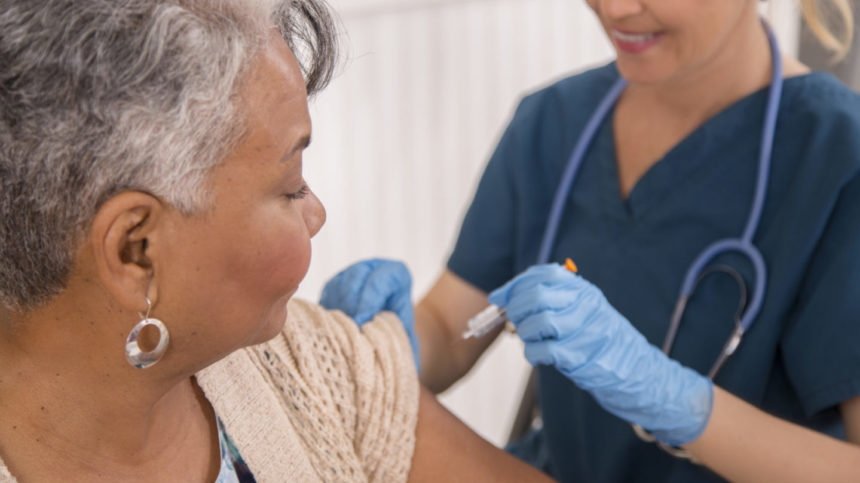
The immunization advisory panel for the Centers for Disease Control and Prevention overwhelmingly affirmed on Tuesday that long-term care residents and healthcare workers will be given first access to a COVID-19 vaccine once authorized by the federal government.
The CDC’s Advisory Committee on Immunization Practices voted 13-1 to approve the groups’ prioritization during an emergency meeting on Tuesday.
There are about 3 million residents at long-term care facilities, which by panel’s definition includes skilled nursing, assisted living and other resident care facilities. There are also about 21 million healthcare personnel. Workers at long-term care facilities, pharmacies and in home health care settings are included in that grouping.
Panel staff members noted that the federal government expects there will be about 40 million doses available to vaccinate 20 million people by the end of December. Officials also anticipate 5 million to 10 million doses per week will be distributed post-authorization.
The advisory board noted that initial doses may be limited and there could be a need for sub-prioritization within the two groups, and the CDC will provide guidance on how to rank within the categories.
For long-term care facilities, those rankings would encourage state officials to consider prioritizing skilled nursing facilities to receive vaccine doses first, then to residents at assisted living facilities, residential care communities, intermediate care facilities and state veterans homes.
For healthcare personnel, considerations called for workers with direct patient contact for top prioritization, followed by workers in long-term care facilities, and employees without any known COVID-19 infection during the prior 90 days.
Panel members also noted that pharmacies, providers and individuals will be encouraged to use various reporting mechanisms — like the Vaccine Adverse Event Report System and the National Health Care Safety Network — to detail any issues or adverse affects of the vaccine.
The sole vote against the recommendation came from Helen Keipp Talbot, M.D., of Vanderbilt University. Talbot expressed skepticism about the lack of vaccine efficacy data for long-term care residents and older adults during recent trials. She added that she has no reservations about healthcare workers taking the vaccines.
“I still struggled with this. This was not an easy vote,” she said. “I really hope this highlights that our [SNFs] are a population that needs lots of vaccines, not just COVID, and we really need to start finding ways of developing and testing these vaccines to prolong quality of life for our long-term care facility residents. I think that’s key.”
The panel’s approval comes following moves by President Donald Trump and federal health officials pledging to give long-term care residents top priority for the vaccine.
Officials in mid-October announced a COVID-19 vaccination distribution program that will provide the medication to long-term care facilities at no cost. About 99% of facilities nationwide have signed up to participate in the program.
Health leaders also expect vaccines to be ready for distribution at long-term care facilities in about two weeks.





