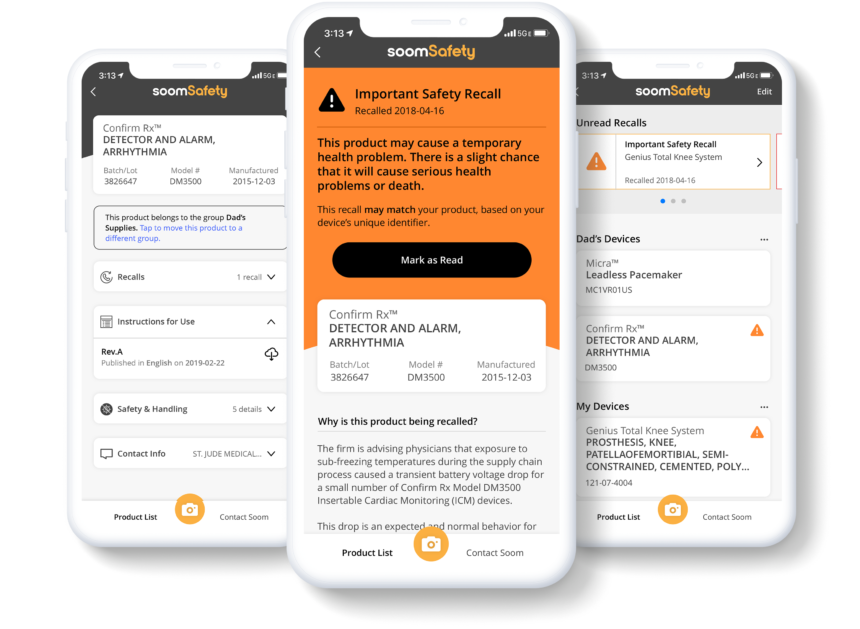
SoomSafety, a new iOS app from Soom, allows users to scan a medical device to receive instructions directly from the manufacturer and the FDA regarding use, safety and recall information.
The app utilizes open-source databases to allow developers to easily access FDA data.
“We built SoomSafety to help patients and caregivers relying on implanted medical devices, and using medical devices at home answer one critical question, ‘Is this medical device safe to use?’” said Charlie Kim, president and CEO of Soom. “Our technology makes it possible to connect previously siloed medical device data, giving patients — and their caregivers — more proactive control over their health and safety.”





