Federal officials Monday provided more guidance to nursing homes on how to conduct daily functions such as masking, testing and resident visits after the COVID-19 public health emergency expires Thursday.
Reminding providers that “visitation is allowed for all residents at all times,” the Centers for Medicare & Medicaid Services updated its nursing home visitation guidance and canceled its August 2020 interim rule governing testing rules for nursing homes.
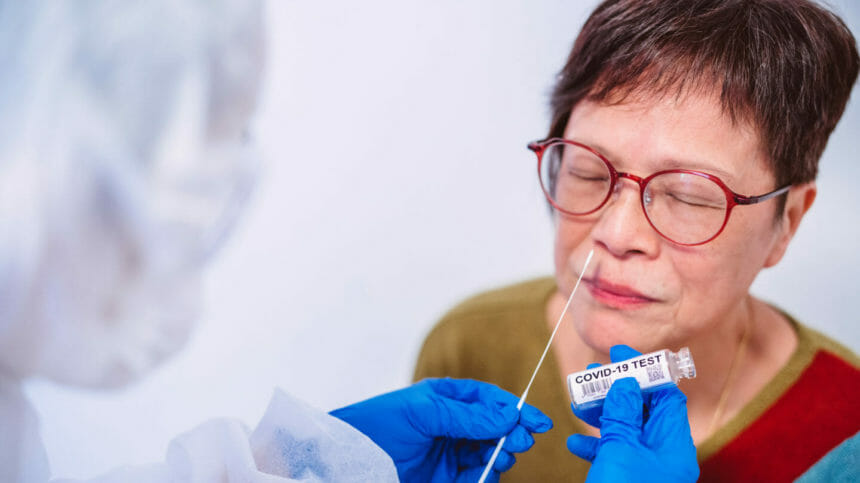
Effective May 11, the COVID testing memo will no longer be in effect. Instead, CMS said providers should comply with “accepted national standards,” such as Centers for Disease Control and Prevention recommendations.
Testing of newly admitted residents and routine testing of asymptomatic staff will both now be at the discretion of facilities. Nursing homes should rely on the Centers for Disease Control and Prevention (see below) and local recommendations to determine when such testing might be appropriate.
Moving forward, noncompliance related to COVID testing will be cited under F-tag 880, a general infection prevention and control tag.
“While the PHE will end, CMS still expects facilities to adhere to infection prevention and control recommendations in accordance with accepted national standards,” the agency said in posting the new surveyor guidance regarding visitation.
To its core principles for infection prevention and control, such as masking and hand hygiene, CMS added two new requirements. The agency said providers should adopt all of its principles regardless of how or where visits are conducted. They should also:
- Post visual alerts such as signs or posters at entrances and in strategic places such as waiting areas, elevators and cafeterias where visitors and entering staff can see them. The alerts should include instructions about current infection prevention recommendations, such as if masking is being used.
- Conduct resident and staff COVID testing following nationally accepted standards, such as CDC recommendations.
During an outbreak investigation, even if only tracking one new case, nursing homes must still allow visits if residents desire them. But CMS recommended that “the visit should ideally occur in the resident’s room, the resident and their visitors should wear well-fitting source control (if tolerated) and physically distance (if possible) during the visit.”
The memo also explicitly noted that visitors can share a meal with or feed the resident they are visiting.
Meanwhile, the CDC posted its own updated recommendations on Monday to help healthcare providers understand how to monitor COVID infections trends with the ending of community transmission measurements on May 11.
“Organizations now need to look to identify local metrics to determine your use of source control, which we know as masking,” explained Janine Finck-Boyle, vice president of Health Services Policy and Regulatory Affairs for LeadingAge. She said she reviewed the CDC guidance just before joining a call with members Monday afternoon.
“You can be using your state and local public health evaluation of any local metrics that exist, or taking any cues from the surrounding community, which could include the COVID-19 hospital admission levels if they are higher.”
When it comes to testing of incoming residents, CDC’s advice is consistent with that of CMS: pre-procedure or pre-admission testing will be at the discretion of the facility for all healthcare settings.




