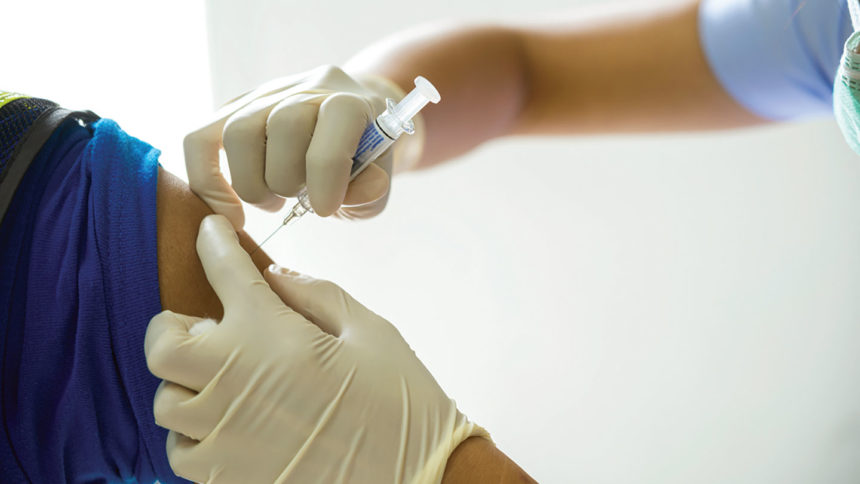
A new study looked at how healthcare workers fared after having a COVID-19 vaccine, a flu shot or both. It found that taking both wasn’t linked to a lower immune response or to more frequent adverse events compared to those who only had a COVID-19 shot. In other words, taking both together is effective and safe, the study published in JAMA Network Open last week said.
The team looked at healthcare workers at a medical center in Israel who got a COVID-19 vaccine, a flu vaccine or both at the same time. They looked at lab samples and questionnaires to evaluate the outcomes. They broke them into two groups for two different analyses: How their immune systems responded and if there were adverse effects.
Compared with COVID-19 vaccination alone, the risk of adverse reactions was similar in the group when people got both vaccines together. The researchers say their findings are similar to other trials that looked at giving both shots together and found that doing so was similar to taking to only the COVID-19 shot.
People who received the flu shot alone had the least immune response. And people who only got the COVID-19 vaccine had a similar reaction to those who got both.
The authors say that knowing coadministration is safe and effective may help more people to get and stay vaccinated.
“Adherence to a single clinic visit will surely be greater than that which can be achieved for two separate visits, especially in more vulnerable populations [like older adults],” the authors wrote.
“Coadministration of vaccines is often advocated when disadvantages are marginal or negligible,” the report said.
The authors say the study was limited because it was mostly conducted on healthy people. They also only tested the immune reaction on SARS-CoV-2 and not the flu.
“Coadministration did not lead to a substantially inferior immune response or to an increased rate of reactogenicity events compared with the administration of this COVID-19 vaccine alone,” the authors wrote.
The Food and Drug Administration on Monday approved new COVID-19 vaccines from Pfizer and Moderna that target another version of omicron, though they say it should work against variants currently circulating. They’re not calling it a booster, though; they say this one specifically aligns with more recent strains of the coronavirus.



