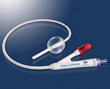
Hospi Corporation has received clearance from the Food and Drug Administration for its Macy Catheter™, which is approved for ongoing rectal delivery of medications and liquids.
The device is particular useful for those with serious or terminal illness who can’t swallow, the company noted.
“Rectal administration is a fast, safe and highly effective route of delivery for many medications, especially those used for management of symptoms at the end of life; however, it is often under-utilized in hospice due to the discomfort and embarrassment associated with suppository administration,” said Brad Macy, RN, BSN, CHPN, co-founder of Hospi.
The catheter increases privacy and allows management of symptom control, he noted.
The Macy Catheter, which is made of silicone, will be available via prescription as a single-use, disposable devices in spring 2014. Once inserted by a clinician, it sits on a patient’s leg or abdomen.




