The Food and Drug Administration has issued an emergency use authorization for an inexpensive, rapid-results coronavirus antigen test that functions like some pregnancy tests.
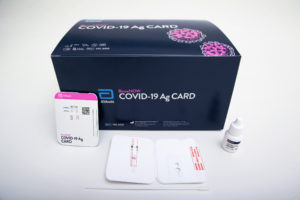
The BinaxNOW COVID-19 Ag Card is intended for individuals suspected of COVID-19 within the first seven days of symptom onset, developer Abbott announced Wednesday. A healthcare provider twirls a nasal swab sample on a credit-card-sized test device with a reagent added. Results can be read directly from the device in about 15 minutes. One line indicates a negative result; two lines indicate a positive result.
Abbott says it will sell the test for $5. No equipment (such as an analyzer) is required to process samples or read test results. The company also offers a complementary smartphone app that allows people to share test results when needed.
The test is a “rapid, reliable, highly portable, and affordable tool for detecting active coronavirus infections at massive scale,” Abbott said. It plans to ship “tens of millions” of the tests in September, ramping to 50 million tests a month in early October.
Rapid antigen testing is also used by COVID testing devices currently being deployed to all U.S. nursing homes by the Department of Health and Human Services. The Quidel Sofia 2 Instrument or a Becton, Dickinson and Company (BD) Veritor Plus System can conduct 10 to 20 tests per hour with results available in 15 minutes, according to Adm. Brett Giroir, M.D., the Department of Health and Human Services’ assistant secretary for health.
Antigen tests in general are “very specific, but are not as sensitive as molecular tests,” the FDA reminded potential users. Negative results may need to be confirmed with a molecular test prior to making treatment decisions, and they should be considered “in the context of clinical observations, patient history and epidemiological information,” the agency stated.




