
Formal training for healthcare providers has traditionally focused on the classic signs of infection. In many cases of chronic wounds, the signs are subtle, and the ability to recognize them can make a big difference in the effective management of a wound.
Pressure ulcers and other chronic wounds remain a challenge in the US. An estimated 1 to 3 million people develop pressure ulcers each year, and 60,000 die from associated complications (1, 2). Many systemic and local factors may affect wound closure. Systemic factors include comorbidities, nutrition, age, stress and tissue oxygenation. Local factors include blood flow, mechanical stress (pressure, friction and shear) and the use of cytotoxic agents (3). A comprehensive assessment of the resident with a chronic wound can help clinicians to identify and address both systemic and local factors.
In addition to the above mentioned factors, the International Advisory Panel on Wound Bed Preparation identified four major barriers to wound closure: non-viable tissue, infection or inflammation, moisture imbalance, and a non-advancing or undermined wound edge (4). It may be easy to recognize necrotic tissue in a wound, a wound that is too wet or too dry, and even a wound in which the edges or undermined areas are not progressing. However, identifying infection in the chronic wound can be difficult.
Part of the problem is that, during formal training, most healthcare providers are taught to assess for only classic signs of infection: erythema, induration, warmth, fever, edema/swelling, foul odor, purulence and pain (5, 6). These classic signs appear in acute wound infection or perhaps in a severely infected chronic wound. With most chronic wound infections, however, there are subtle, and therefore often overlooked, secondary signs and symptoms, which occur more often than the classic signs. These secondary sign and symptoms include delayed healing, change in color of the wound, friable (bleeds easily) granulation, absent or abnormal granulation, odor, breakdown of the wound and pain (7).
Wound cultures
The topic of wound cultures is controversial. Although used for more than 100 years, a discussion of swab cultures generates debate over appropriate swab type, technique, whether surface organisms reflect deeper wound pathogens and whether certain pathogens may be missed (6).
Quantitative cultures, considered the gold standard by many, require tissue samples and provide a count—or quantity—of the pathogens. Used for more than 40 years, this culture technique is traumatic, expensive and typically requires specific skill and licensure. While delayed healing is associated with greater than 105 colony-forming units per gram of tissue in a number of wound types, 20% of wounds with this number of bacteria will close. Additionally, small quantities of some virulent bacteria may delay healing. Infection, therefore, may be more about the resident’s ability to “fight” infection than a number from a lab test. Delayed healing from bacteria occurs when the amount and/or type of bacteria overpower the resident’s immune function (6).
Wound cultures should be obtained after clinical diagnosis of infection. Nearly all wound cultures will be positive because all wounds are 100% contaminated (bacteria are present) following injury (6). But if the wound is progressing, bacteria in that wound is not causing a problem.
The contamination-infection continuum
Carville et al (8) offered a contamination-infection model for assessing and treating wounds, based upon bacterial presence (see below).
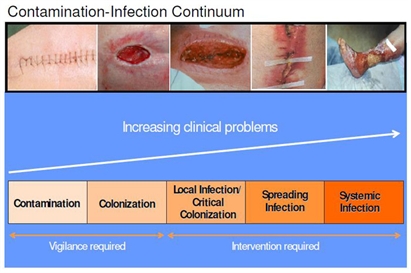
Contamination is the presence of non-replicating bacteria which does not delay wound closure. Colonization means that bacteria are replicating but still pose no problems for the host. Critical colonization, also called local infection, is a relatively new concept to describe a wound that has stopped progressing due to bacterial burden (9). Critically colonized wounds may exhibit those secondary signs and symptoms of infection, discussed earlier. Although classic signs of infection are not present, the wound does not progress.
Spreading infection is identified by the classic signs of infection (8). If allowed, local or spreading infection may become systemic, resulting in fever, chills, hypotension, organ failure and death (6).
All wounds exist in a state between contamination and infection. Determining a resident’s position on the continuum is important for choosing a plan of care. If the wound is progressing, it is most likely contaminated or colonized. Cleansing and appropriate dressing selection may be the only management requirements. If the wound exhibits signs of local infection or critical colonization, a management plan may include cleansing, debridement, if necessary, and the use of antibacterial dressings.
Two types of contemporary antibacterial dressings are those with nanocrystalline silver, and those with cadexomer iodine. Unlike traditional silver dressings (silver nitrate and silver sulfadiazine cream), which require frequent application, some newer silver dressings provide bactericidal levels of silver for up to seven days. And unlike traditional iodine solutions, which may contain 5-10% iodine, cadexomer iodine contains 0.9% iodine in a cadexomer bead. In addition to reducing the bacterial burden, cadexomer iodine absorbs exudate and removes loose slough and debris. It may be left in the wound up to 72 hours.
Individuals with spreading or system infection require systemic antibiotics. However, systemic antibiotics may not reach therapeutic levels in some wounds (10). So, although an individual may be receiving systemic antibiotics, it may be important to continue management with antibacterial dressings too.
A comprehensive assessment of the resident, the environment and the wound, is vital to successful wound management. And recognizing wound infection is an important part of the assessment. While signs and symptoms of chronic wound infection can be subtle, a clinician who can identify and act upon those often overlooked signs can make a big difference in the progress of residents with wounds.
References
1. Dorner B, Posthauer ME, Thomas D. The Role of Nutrition in Pressure Ulcer Prevention and Treatment. Nutrition White Paper. National Pressure Ulcer Advisory Panel. 2009. www.npuap.org.
2. Park-Lee E & Caffrey C. Pressure Ulcers Among Nursing Home Residents. U.S. Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics. Data brief # 14. February, 2009.
3. Doughty D & Sparks-Defriese B. In Bryant & Nix (Eds.) Acute and Chronic Wounds: Current Management Concepts (3rd Ed). Mosby: St. Louis
4. Schultz G. Sibbald RG, Falanga V, Ayello A, Dowsett C, Harding K, Romanelli M, Stacey M, Teot L, Vanscheidt W. (2003) Woud bed preparation: a systematic approach to wound management. Wound Repair & Regeneration 11(1): 1-28.
5. Cutting K & White R. British Journal of Community Nursing. 2004; 9(3 Suppl); S6–S16.
6. Dow G. Bacterial Swabs in the Chronic Wound: When, How and What do They Mean. Ostomy/Wound Management – ISSN: 0889-5899 – Volume 49 – Issue 5A – May 2003 – Pages: 8 – 13
7. Gardner S & Frantz R. In Baranoski & Ayello (Eds). Wound Care Essentials: Practice Principles (2004) Lippincott, Williams & Wilkins; Philadelphia.
8. Carville, K et al 2008 Wound Infection in Clinical Practice: An international consensus. International Wound Journal Vol. 5, supplement 3. pp 1-11.
9. Stotts N. In Bryant & Nix (Eds.) Acute and Chronic Wounds: Current Management Concepts (3rd Ed). Mosby: St. Louis
10. Robson MC, Edstrom LE, Krizek TJ, et al. The efficacy of systemic antibiotics in the treatment of granulating wounds. J Surg Res. 1974;16:299-306.
Terry Coggins, MSN, RN, WOCN, CWCN, CWCN, is medical education manager for Smith & Nephew Wound Management.



