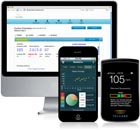
Telcare has received Food and Drug Administration approval for its Telcare BGM 3G blood glucose meter. The product acts like a normal glucose meter, but embeds cellular connectivity that sends blood glucose data to its servers. The device uses the broadband spectrum to transmit data economically and instantaneously without requiring cellular voice and data contracts for operation. Telcare is also developing a suite of smartphone apps for caregivers such as relatives of diabetic seniors.



